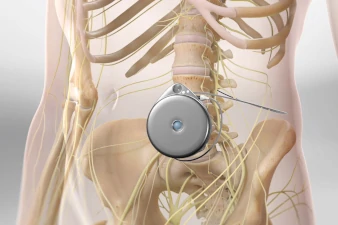
What is an Intrathecal Pain Pump?
The Intrathecal Pain Pump procedure, a sophisticated pain management solution, involves two key phases: a trial period followed by the surgical implantation of a pump that delivers medication directly into the intrathecal space around the spinal cord. This trial is critical, allowing both the patient and the healthcare provider to assess the effectiveness of the medication (such as opioids or antispasmodics) in managing chronic pain conditions or spasticity with minimal side effects. The trial involves the temporary administration of medication through a catheter to ensure the patient responds favorably. If successful, the permanent pump is implanted under the skin of the abdomen in a subsequent procedure, with a catheter that extends to the intrathecal space.
This permanent implantation is performed under general anesthesia. The pump, a programmable device, is then set to deliver the prescribed medication dosage at scheduled intervals, directly targeting the spinal cord's pain-transmitting nerves. The ability to adjust the pump externally allows for tailored pain management, adapting to the patient's evolving needs. By providing a direct delivery of medication, the Intrathecal Pain Pump procedure offers an effective alternative for patients whose chronic pain has not responded to other treatments, significantly enhancing their quality of life through improved pain control.
What is a Pain Pump Trial?
A pain pump trial, also known as an intrathecal pump trial, is a medical procedure used to determine if a patient will benefit from long-term delivery of medication directly into the spinal fluid to manage chronic pain. This trial involves the temporary administration of medication through a catheter placed in the intrathecal space around the spinal cord. The process typically lasts for a few days, during which the patient is monitored to assess the effectiveness of the medication in reducing pain levels without causing unacceptable side effects. If the trial proves successful, indicating significant pain relief and improved quality of life, a permanent intrathecal pump may be implanted. This device delivers a controlled dose of medication directly to the spinal cord, offering a targeted approach to pain management that can reduce the side effects often associated with oral pain medications.
What are the benefits and risks of a Pain Pump?
For many patients with chronic pain who have not found relief through other treatments, a pain pump can offer a valuable option for managing their pain and improving their quality of life. Schedule a consultation with us to discuss the potential benefits and risks to make an informed decision about this pain management approach.
Benefits
- Effective Pain Management: Pain pumps deliver medication directly to the intrathecal space around the spinal cord, providing significant pain relief more effectively than oral medications for many patients.
- Reduced Systemic Side Effects: Because the medication is delivered directly to the site where pain signals are transmitted, lower doses are required, minimizing the systemic side effects often seen with oral pain medications.
- Adjustable Dosage: The pump allows for precise control over the amount of medication delivered, enabling customization of the pain management regimen to the patient's specific needs. Adjustments can be made relatively easily by a healthcare provider.
- Improved Quality of Life: For many patients, pain pumps lead to a substantial improvement in quality of life. They can experience reduced pain levels, which may allow for increased mobility, better sleep, and the ability to participate more fully in daily activities and physical therapy.
- Continuous Pain Relief: The pump provides a continuous delivery of pain medication, ensuring consistent pain management without the fluctuations in pain levels that can occur with oral medications.
Risks
- Surgical Risks: The implantation of a pain pump is a surgical procedure, carrying risks such as infection, bleeding, and, in rare cases, spinal fluid leak.
- Device Complications: Complications with the pump or catheter, such as malfunction, blockage, or dislodgement, can occur, potentially requiring additional surgery to repair or replace the device.
- Medication Side Effects: Despite the targeted delivery, patients may still experience side effects from the medication, including nausea, dizziness, constipation, and urinary retention. There's also a risk of overdose if the pump malfunctions or if there's an error in the dosage settings.
- Dependence and Tolerance: Over time, patients may develop tolerance to the medication, requiring higher doses to achieve the same level of pain relief. There's also a risk of psychological dependence on the device for pain management.
- Infection: There's a risk of infection at the site of the pump implantation or in the intrathecal space, which can be serious and require treatment with antibiotics or surgery.
- Cost and Maintenance: Pain pumps and the required ongoing medication refills can be costly. Additionally, the pump requires regular maintenance, including refilling the medication reservoir and periodic battery replacement for the pump, which may require additional surgeries.
It's crucial for individuals considering an SCS to discuss these potential benefits and risks with a specialist. During your pain management consultation, we will discuss your specific condition, medical history, and lifestyle to determine whether an SCS is a suitable option for your pain management needs.
Who is a good candidate for a Pain Pump procedure?
A good candidate for a pain pump procedure, also known as intrathecal drug delivery system implantation, typically meets several criteria indicating that they would benefit significantly from this form of pain management. These criteria include:
- Chronic Pain: The individual suffers from chronic pain that is not adequately managed by oral medications, physical therapy, or other less invasive treatments.
- No Surgical Options: The pain is not amenable to surgery or the individual has already undergone surgery without sufficient relief.
- Successful Pain Pump Trial: The individual has undergone a trial period with a temporary intrathecal pump and experienced significant pain relief without adverse side effects.
- No Psychological Contraindications: The individual has been evaluated to ensure there are no psychological conditions or issues that might contraindicate the implantation of a pain pump or affect its success.
- Good Overall Health: Apart from their chronic pain condition, the candidate is in relatively good health, with no medical contraindications to the surgery required for implanting the pump.
- Understanding and Compliance: The individual understands the nature of the procedure, the need for regular follow-up visits for pump refills and adjustments, and is committed to adhering to the treatment plan.
Candidates often suffer from conditions such as severe spasticity due to multiple sclerosis, chronic back pain after failed back surgery, cancer pain not relieved by other treatments, or neuropathic pain. A pain pump can offer a more effective and targeted method of pain management for these individuals, significantly improving their quality of life. However, the decision to proceed with a pain pump implantation involves careful consideration of the potential benefits and risks, as well as a thorough discussion between the patient and a specialist.
How is a Pain Pump procedure performed?
Here's a general overview of the process:
Trial Phase
- Pre-Trial Assessment: The patient undergoes a thorough evaluation, including medical history, physical examination, and sometimes imaging studies, to confirm that they are a suitable candidate for the procedure.
- Consent and Preparation: Informed consent is obtained, explaining the risks and benefits of the trial. The patient might be advised to stop certain medications before the procedure to reduce the risk of bleeding.
- Catheter Placement: The trial usually takes place in a hospital or outpatient setting under local anesthesia, sometimes with sedation. The doctor inserts a thin, flexible catheter into the intrathecal space of the spine using fluoroscopy (real-time X-ray) to guide the placement.
- Medication Administration: Once the catheter is in place, medication (typically an opioid, local anesthetic, or a combination) is administered through the catheter to the intrathecal space. The type and dosage of medication are carefully selected based on the patient’s specific pain management needs.
- Monitoring and Assessment: The patient is monitored for several hours to days to assess the effectiveness of the pain relief and to monitor for any adverse reactions. The patient and healthcare provider will discuss the level of pain relief experienced and any side effects encountered.
Permanent Pump Implantation (If Trial is Successful)
- Surgical Procedure: Performed under general or spinal anesthesia, the procedure involves making a small incision in the back to place the catheter into the intrathecal space, similar to the trial phase. Another incision is made in the abdomen to implant the pump itself, a programmable device that stores and delivers medication.
- Pump Programming and Filling: The pump is then programmed to deliver the prescribed amount of medication. It can be adjusted or refilled with medication as needed, through an outpatient procedure.
- Recovery: The patient will have a recovery period where they may need to limit certain activities to allow the incision sites to heal properly. Pain relief and pump function are closely monitored, and follow-up appointments are crucial for optimal management.
Important Considerations
- Trial Success: A successful trial is one where the patient experiences significant pain relief without intolerable side effects. It’s a crucial step in determining the efficacy of intrathecal pain management for the individual.
- Commitment to Follow-up: Patients must commit to regular follow-up visits for pump refills, monitoring, and adjustments.
This procedure represents a significant intervention in pain management for patients with chronic pain conditions, especially those who have not responded to other treatments. It offers targeted pain relief with reduced systemic side effects but requires careful consideration, given the invasiveness and need for ongoing management.